Which of the Following Liquids Has the Highest Surface Tension
Water oil bromine mercury. 6 Why does water have a strong surface tension and why is this important.
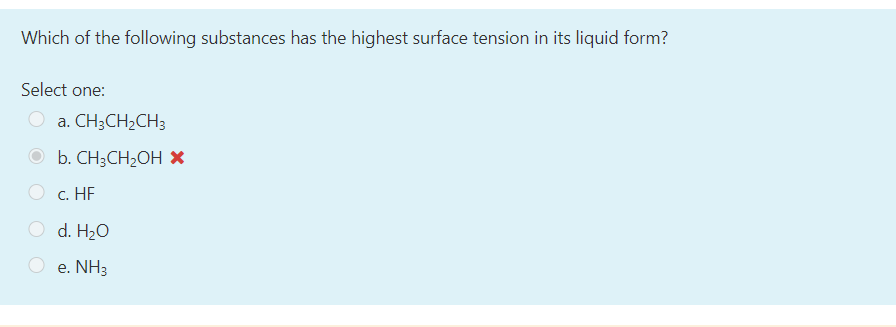
Solved Which Of The Following Substances Has The Highest Chegg Com
Water oil bromine mercury water oil bromine mercury Weegy.

. Glycerol in water has the highest surface tension. Which of the following liquids has the maximum surface tension at a given temperature. It is the result of intermolecular forces.
The surface tension of soap in water and detergent in water is lower than the surface tension of pure water. 5 What characteristic allows water to have a high surface tension. Sparingly soluble salts and surface active substances decrease the surface tension of the liquid however the fairly soluble solutes increase the surface tension of the liquid.
Option c is correct Explanation. Get started for FREE Continue. Attachment of these molecules with each other gives rise to surface tension.
Rank from greatest to least surface tension. Please check contributions posted by others. 100 correct and accurate.
Attachment of these molecules with each other gives rise to surface tension. The water molecules attract one another due to its polar property the hydrogen ends which are positive in comparison with the negative end of oxygen which makes water to stick together so water having maximum surface tension. Which of the following liquid substances would you expect to have the lowest surface tension.
It is due to surface tension the insects float on a liquid. Which of the following liquids has the highest surface tension. Which of the following liquids has the highest surface tension.
Option A Explanation No official explanation is available for this question at this time. However the fairly soluble solutes increase the surface tension of the liquid. So among the given options Glycerol in water has the highest surface tension because glycerol has more hydrogen bonds formed per molecule.
Which of the following liquids is likely to have the highest surface tension. The containers volume is increased to 20 L at constant temperature and the liquidvapor equilibrium is reestablished. 4 Why does water have high surface tension but low viscosity.
Water has highest surface tension. What Liquid has the Strongest Surface Tension. 3 Why does water have more surface tension than other liquids.
A Pb B CH3OCH3 C HOCH2CH2OH D H2O E CH3CH2OH. Which of the following liquid shows highest surface tension. What you will need.
Molecules at the surface are strongly attracted to the molecules below. Amount of each liquid used Weights used to measure surface tension Temperature of liquid Weights- I used small pieces of lead Cup for holding the liquid. See below for the correct answer.
Which of the following has the highest surface tension. Molecules at the surface are moderately attracted to the molecules below. Which of the following does not affect surface tension of.
Water has highest surface tension. The water has the highest surface tension. Option c is correct.
Which of the following liquids will have the highest surface tension. 100 10 ratings Amongst the given optionsWater has highest surface tensionSurface tensi. Which of the following liquids has the highest surface tension.
Mercury has the highest surface tension. The water has the highest surface tension. Salty water Correct Answer.
There are molecules present in every liquid. Which of the following liquids has the highest surface tension. 7 What causes the high surface tension low vapor pressure and high boiling point.
Expert answered allmost Points 46. Bob mentioned to me before surface tension is how the surface molecules stick together. Mercury water oil or bromine Mercury has the highest surface tension.
3 A sample of octane in equilibrium with its vapor in a closed 10 L container has a vapor pressure of 500 torr at 45C. A Br2 B C8H18 C CH3OCH3 D CH3OH E Pb. There are molecules present in every liquid.
View the full answer. We review their content and use your feedback to keep the quality high. Rank these liquids by their expected surface tension.
Which of the following factors contributes to a low viscosity for a. It is the result of intermolecular forces. 1-propanol CH CHCH OH b.
Question 6 of 10 Which statement describes a liquid that has high surface tension. Molecules at the surface are weakly attracted to the molecules below. Which of the following has the highest surface tension.
Experts are tested by Chegg as specialists in their subject area. It is due to surface tension the insects float on a liquid.

Solved Which Of The Following Liquids Has The Highest Chegg Com
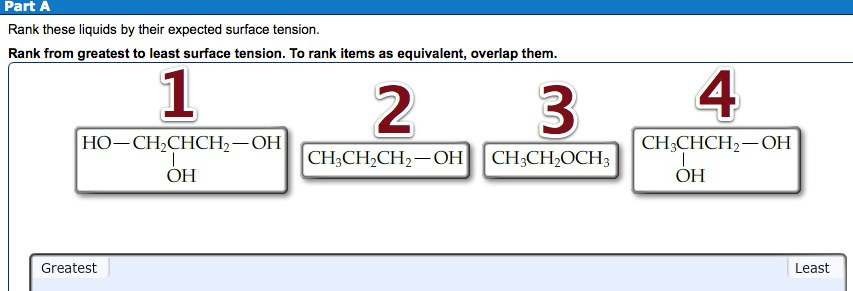
Rank These Liquids By Their Expected Surface Tension Rank From Greatest To Least Surface Tension Home Work Help Learn Cbse Forum
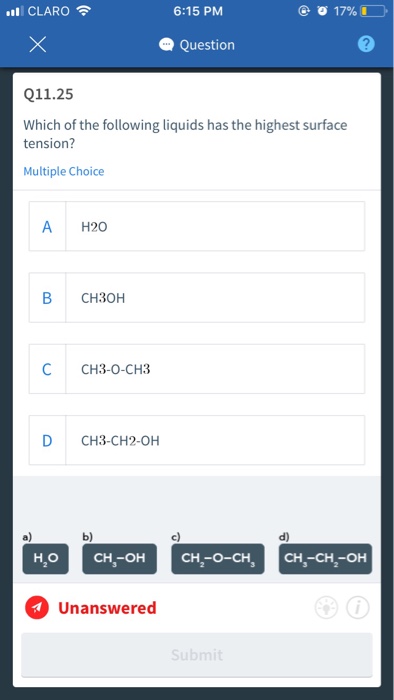
Solved Claro 6 15 Pm Question Q11 25 Which Of The Following Chegg Com
Comments
Post a Comment